
Descriptive vs Narrative Essays
The IGCSE English First Language exam places a strong emphasis on creative writing, with writing descriptive and narrative essays being
Learn how to write step-by-step answers, and score A* in your exam!

Announcement: Cambridge IGCSE, and AS & A Level November 2024 past papers are now available.
In Chemistry, writing a balanced chemical equation is a skill that needs practice. This article will discuss all the tips and tricks to hone that skill.
A chemical reaction is the process of changing reactants into products. A chemical equation, however, is a symbolic representation of that reaction. Chemical equations denote the molar quantities and the identities of different reactants and products involved in the reaction.

The reactants are written on the left-hand side, while the products are represented on the right-hand side of an equation. The arrow shows the direction in which the chemical change occurs. More than one reactants or products on either side of the equation are separated by using a plus (+) sign between them:

The most common reaction arrow points from left to right (→), symbolising the reactants changing into products or the reaction occurring in the forward direction.
A balanced chemical equation has an equal number of atoms of each element on both sides of the reaction. This concept is based on the law of conservation of mass, i.e., mass cannot be created or destroyed; it can only be converted from one form into another where the total mass of the system stays constant.
An equal number of atoms from the same elements are present on both sides of a balanced chemical equation, only their combination changes in transforming from reactants to products. Remember, no elements appear on one side of the equation and not on the other side.
I’ve learned advanced things much faster than a teacher could have taught me
“I am really enjoying Skolatis! It is very helpful. I feel that the way you teach the subjects are very helpful and aid in helping people learn more complicated things. For example I’m a lot ahead in chemistry and biology. I’ve learned many more advanced things much faster than a teacher could have taught me it. Skolatis has really helped me improve in chemistry and biology.”
~ James Armenis (Greece)
Here are the 5 key steps in writing a balanced chemical equation:
We will take one example reaction:
Magnesium metal reacts with hydrochloric acid to form magnesium chloride salt and liberate hydrogen gas.
In this reaction, we have identified:
Reactants = magnesium and hydrochloric acid.
Products = magnesium chloride and hydrogen.
magnesium + hydrochloric acid → magnesium chloride + hydrogen
Mg + HCl → MgCl2 + H2
Stoichiometric coefficients are added with the reactants and products to equalise the number of atoms of each element present on both sides.
In this example, there is 1 Mg-atom on both sides of the equation; hence, it is balanced. However, there is 1 Cl-atom and 1 H-atom on the left side, while 2 Cl-atoms and 2 H-atoms on the right side. To balance, we need to add the coefficient 2 with HCl.
Mg + 2HCl → MgCl2 + H2
Now, there are 2 Cl-atoms and 2 H-atoms on both sides. In this way, it is a fully balanced chemical equation.
Note:
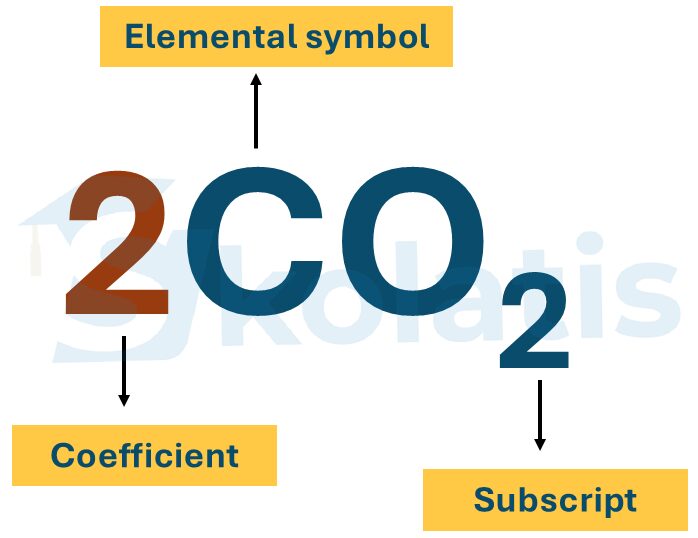
Stoichiometric coefficients are the numbers added at the front of the chemical formulae that denote the number of atoms of each element in the molecule. For example, 2CO2 represents 2 carbon dioxide molecules, meaning 2 carbon atoms and 4 atoms of oxygen.
Remember, while balancing chemical equations, we can change the stoichiometric coefficients but not the subscripts of the chemical formula because that would mean changing the chemical composition itself. For example, O2 is oxygen, but O3 denotes the ozone gas.
Mg(s) + 2HCl(aq) → MgCl2(aq)+ H2(g)
This step is optional, depending on whether you are asked to write the state symbols in the question statement or not. If not, it is better to skip the state symbols.
I recommend it to anyone who wants excellent results
“Words cannot describe how much it has helped me. I recommend it to anyone who wants excellent results. Give it a shot!”
~ Tino Mutangadura (South Africa)
Balance the chemical equation for the reaction of iron (Fe) with oxygen (O2) to form iron (III) oxide.
Step 1:
Identify the different reactants and products.
Reactants: Iron (Fe) and oxygen (O2)
Product: Iron (III) oxide (Fe2O3)
Step 2:
Write the equation in words.
Iron + oxygen → Iron (III) oxide
Step 3:
Convert words into chemical formulae.
Fe + O2 → Fe2O3
Step 4:
Add Stoichiometric coefficients.
Only 1 Fe-atom on the reactant side but 2 Fe-atoms on the product side, so add the coefficient 2 in front of Fe.
2Fe + O2 → Fe2O3
Now, there are 2 Fe-atoms on both sides.
2 O-atoms on the reactant side but 3 O-atoms on the product side, so add the coefficient 3 in front of O2 and 2 in front of Fe2O3.
2Fe + 3O2 → 2Fe2O3
Now, there are 6 O-atoms on both sides. However, it disturbed the balance of Fe-atoms as there are now 4 Fe-atoms on the product side but only 2 Fe-atoms on the reactant side. So, change the coefficient 2 with 4 in front of Fe.
4Fe + 3O2 → 2Fe2O3
In this way, it is a fully balanced chemical equation with 4 Fe-atoms and 6 O-atoms on both sides:
4Fe + 3O2 → 2Fe2O3
This example shows that balancing a chemical equation is just a matter of finding the right coefficients. The choice of the correct coefficients comes with practice, similar to a trial and error method. Moreover, the coefficients can be changed from either or both the reactant and product sides in order to equalise the number of different atoms present.
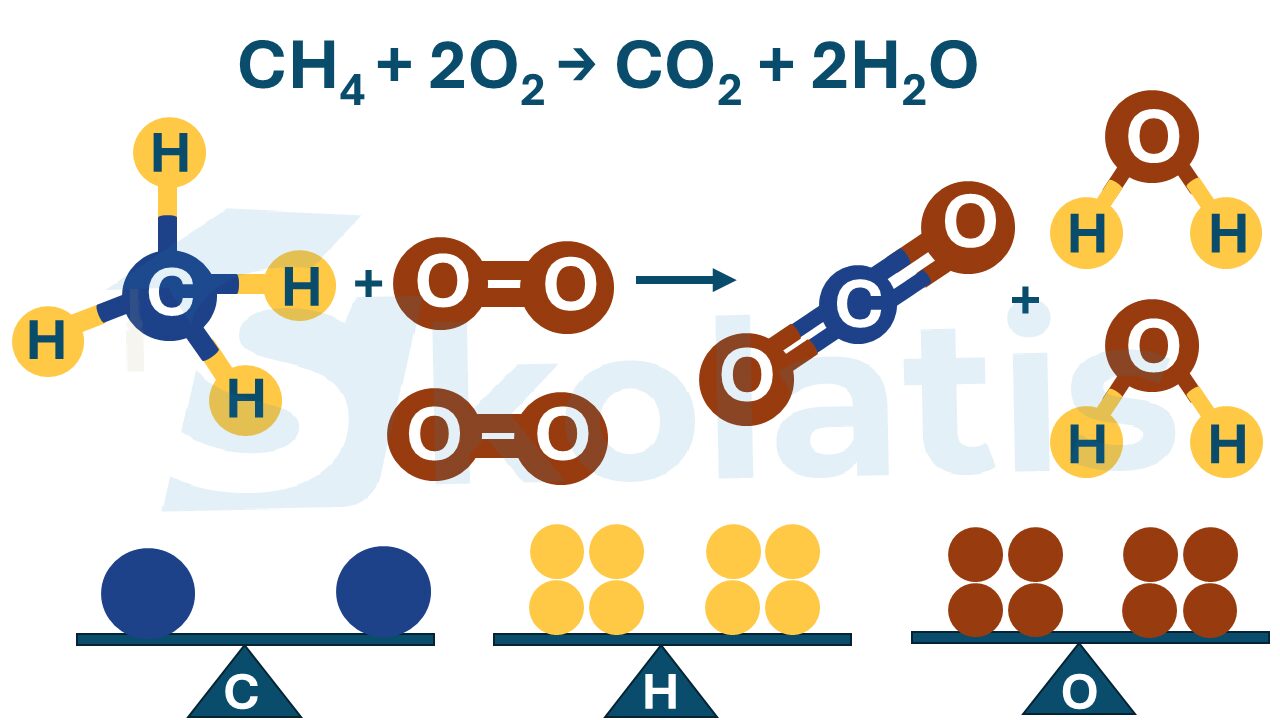
Balance the chemical equation for the complete combustion of pentane that produces carbon dioxide and water.
Step 1:
Identify the different reactants and products.
Reactants: pentane (C5H12) and oxygen (O2)
Products: carbon dioxide (CO2) and water (H2O)
Step 2:
Write the equation in words.
pentane + oxygen → carbon dioxide + water
Step 3:
Convert words into chemical formulae
C5H12 + O2 → CO2 + H2O
Step 4:
Add Stoichiometric coefficients
5 C-atoms are on the reactant side while 1 C-atom is on the product side, so add the coefficient 5 in front of CO2.
C5H12 + O2 → 5CO2 + H2O
Now, there are 5 C-atoms on both sides.
12 H-atoms on the reactant side while 2 H-atoms on the product side, so add the coefficient 6 in front of H2O.
C5H12 + O2 → 5CO2 + 6H2O
This makes a total of 12 H-atoms on both sides.
2 O-atoms on the reactant side while a total of 5(2)+6 = 16 O-atoms on the product side, so add the coefficient 8 in front of O2.
C5H12 + 8O2 → 5CO2 + 6H2O
Now, there are 16 O-atoms on both sides.
So, the fully balanced chemical equation is:
C5H12 + 8O2 → 5CO2 + 6H2O
Balanced chemical equations ensure that the fundamental law of conservation of mass is followed. They provide the correct ratio of reactants and products, which is essential for determining the amounts of substances involved in a chemical reaction. So many parts of Chemistry depend on this one skill; therefore, make sure you master the skill of writing balanced chemical equations to ace your exams.
Find out in our A* Model Answers. Copy the style and score A*!
Come join our A* revision courses that are designed based on proven A* scoring system, move up your level and reach your A*!
Join 60,657 (and counting) IGCSE & AS/A Level subscribers who’ve taken our insanely valuable FREE email courses. Learn exam tips & score A* in your exam!
Abhishek Bathina
Abhishek Bathina
Raunaq Nambiar
Raunaq Nambiar
Jyoti
Jyoti
Soham Santra
Soham Santra
Yusra
India
Yusra
India
Christina Theosabrata
Christina Theosabrata
Harsh Aryan
Harsh Aryan
Akshit Philip
Bahrain
Akshit Philip
Bahrain
Eva Muthusamy
Malaysia
Eva Muthusamy
Malaysia
Lanja Omer
United Arab Emirates
Lanja Omer
United Arab Emirates

The IGCSE English First Language exam places a strong emphasis on creative writing, with writing descriptive and narrative essays being

In the IGCSE English First Language Directed Writing task, you’ll be required to either write a speech, letter, or article

In the face of violence and uncertainty, education stands as a beacon of hope, offering students the tools to navigate

IGCSE stands for “International General Certificate of Secondary Education.” It is an internationally recognised qualification for secondary school students, typically

Our best-selling exam essentials.

.